Preface
Since the early 1900’s it has been well understood that we, as marketers, sell holes, not drills. Translating that adage into the 2000s and relating it to medical devices, the saying goes something like this; we sell improved clinical outcomes, not the tool that provides it. We sell greater safety, not the equipment that enables it. Yet, I still see advertisements that describe in great detail the physicality of the device. I hear sales representatives take about how their device is made of optically clear plastic.
We complain that it takes a decade for physicians to adopt our technology, and yet, we take decades to deploy the latest realizations in medical device marketing.
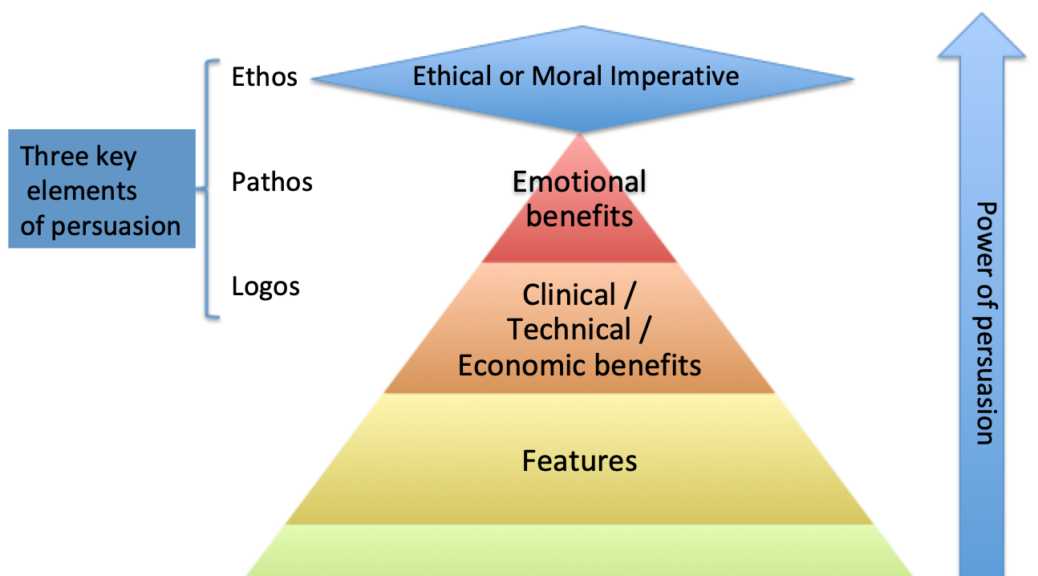
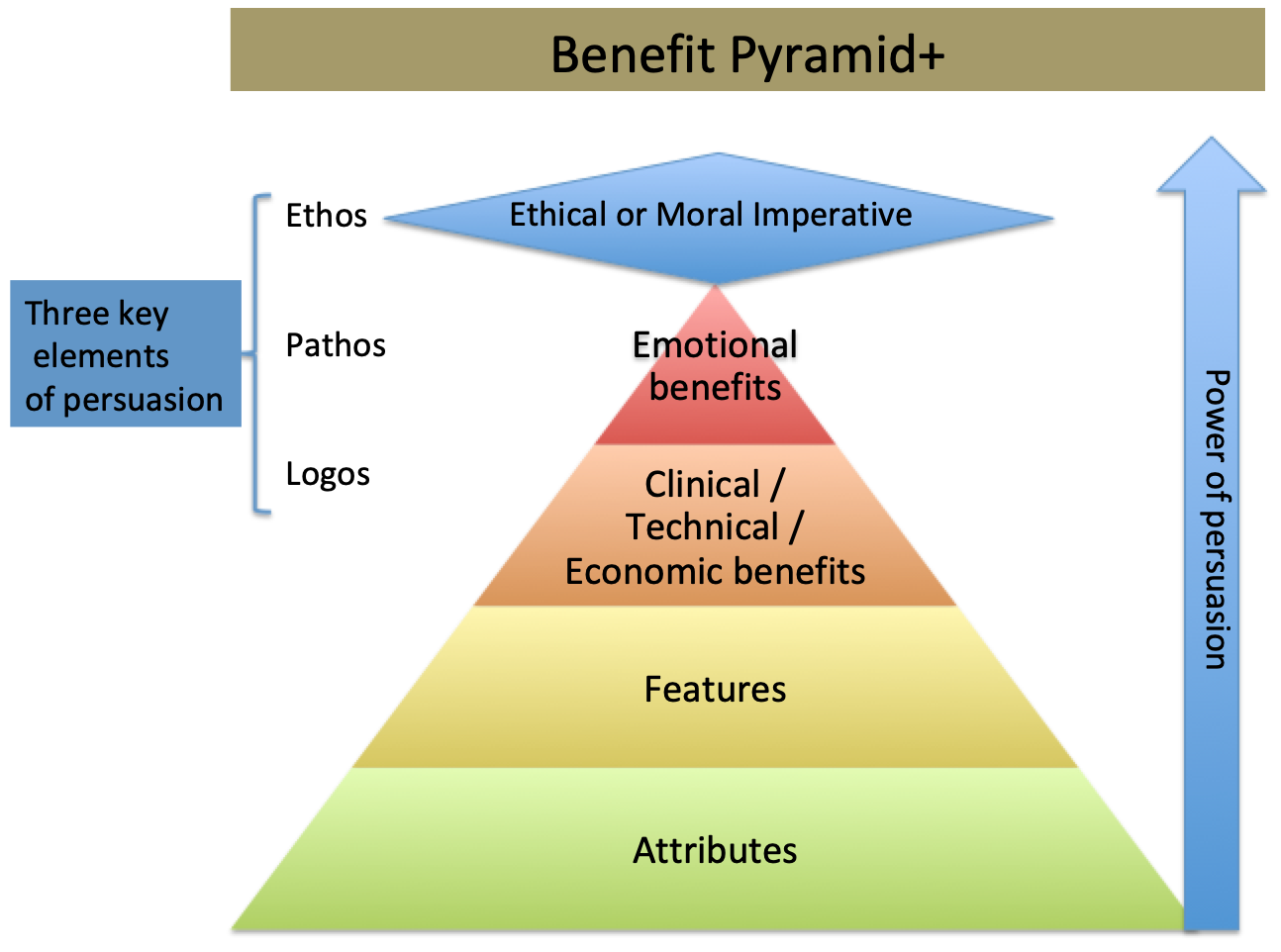
Virtual or not, digital or not, regardless of the channel, we must deliver a clear and relevant message. We must promote the outcomes of our products, not what the product is. The clinical benefits and health economic benefits motivate the healthcare institution to purchase, not the device’s features and attributes. They never get to what clear empowers or enables. They let the clinician connect the dots.
Set-up
Recently, I was working with a client on developing the messaging for a new medical device. He went on for 15 minutes on what the product was, how it worked, its cost, how it was better, lighter, and faster than the competition. He was articulate and passionate. It was truly amazing to hear.
When he finished, I asked a couple of simple questions. Why would I need your device? What will your device allow me to accomplish? This sparked another 15-minute download about the therapy that the device supported. He was equally articulate about the therapy description as he had been about the device description.
He started to realize that the connection between the two revealed the benefit that the device brought; that is what we were selling.
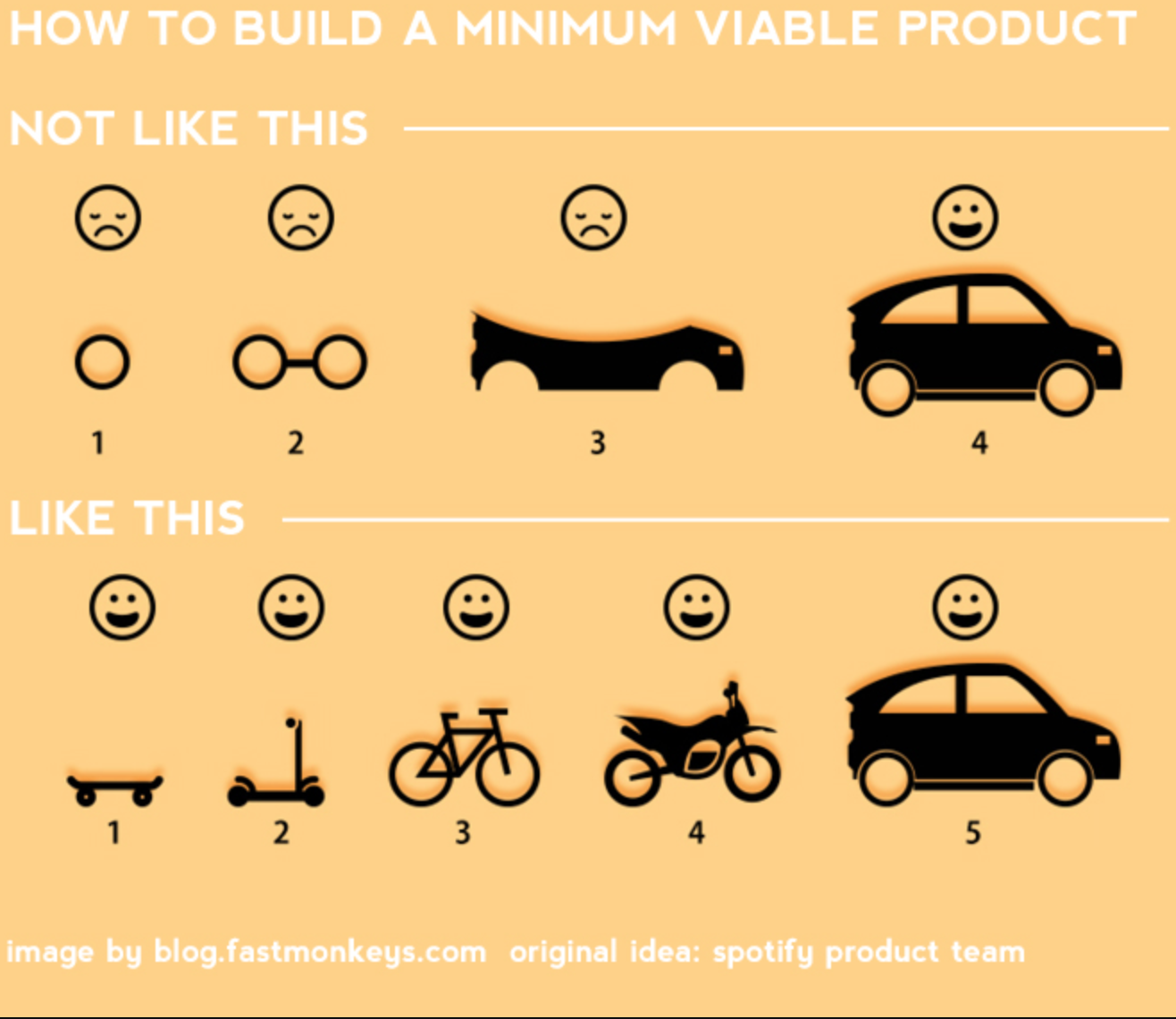
The features and attributes exemplify how our drill makes the hole better than our competition’s.
I pulled this quote out and had him read it; then, I watched as the wheels turned.
Leo McGivena: “Last year one million quarter-inch drills were sold, not because people wanted quarter-inch drills, but because they wanted quarter-inch holes. . .”
How do you get to the right message?
-
- We started with a one-paragraph description of the clinical problem from the perspective of the clinician.
- (Why we needed a hole)
- We then wrote a two-paragraph product positioning (benefit) statement.
- (How we provided the hole.)
- We then wrote a one-page value proposition.
- (Why our hole was more valuable than the hole our competitors made.)
- We then crafted a pricing strategy.
- (What was the realizable value of the hole.)
- We started with a one-paragraph description of the clinical problem from the perspective of the clinician.
Note: These documents are defined in previous posts and in INSIGHTS: 33 lessons learned in medical device marketing available on Amazon.
Result
Over the course of 3-weeks, we had created four of the five foundational documents (messages) that are needed to drive all the down-stream marketing activities and collateral material.
Lesson(s)
1. Crafting a message is critical, no matter how long it takes.
2. Creating great foundational work is crucial to successful communication with the clinician customer.
3. Who delivers the message, how the message is delivered, where the message is delivered, are all important, but are secondary to what the message is.
“Experience is what you get, right after you needed it most.”
Make it a great day!
Tim Walker
Tim Walker is the Principal Consultant for The Experia Group, a consulting firm, which provides experience and expertise during critical device commercialization phases to increase the probability of success. Author of INSIGHTS: 33 lessons learned in medical device marketing, available on Amazon.
One-on-one or team coaching is available.
www.theexperiagroup.com. Contact The Experia® Group for a free 30-minute consultation to determine if 30-years of experience can contribute to your success. [email protected].
© 2020, The Experia Group, LLC