Set up
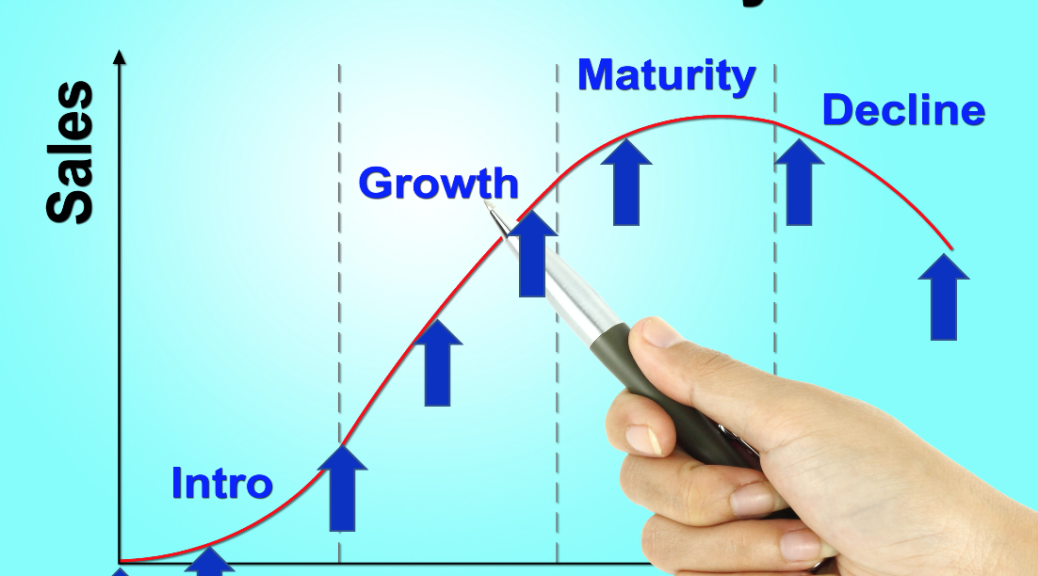
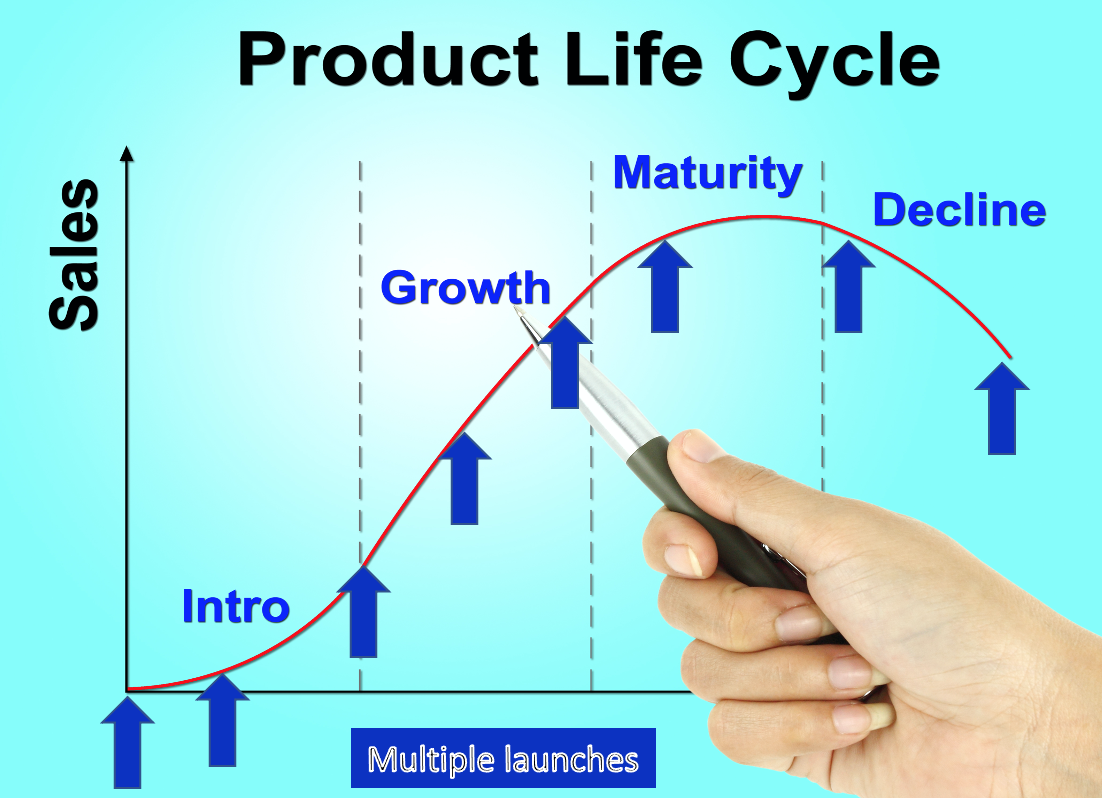
In a coaching session that I held yesterday with a Sr. Product Manager we were preparing for his first-ever new product launch when, it occurred to me that in all the blog posts and in my book, INSIGHTS: 33 Lesson Learned in Medical Device Marketing[1] I have never really explained that a new product launch is not a single event. The launch never really ends. It is made up of events that track the product life cycle. So, it might serve us better to think of them as a series of Launch Campaigns. Across the life cycle, your device will be exposed to different target customers, geographies, cultures, and healthcare systems. Your messaging, channel, proof points all will need to evolve to keep pace with the various clinical evidence changes, new personas, environmental shifts, and learnings that have taken place with time. The goal is to optimize the strategic product message for the point you find yourself in the product life cycle, optimizing lifetime revenue.
Purpose
The purpose of this blog post is to broaden your thinking about the messaging evolution that might be required to facilitate the optimization of adoption at each stage in the product life cycle. To start, what might be some of the indicators that trigger an evolution of messaging.
The first three triggers are obvious ones, but if you don’t have metrics in place you might miss a trigger.
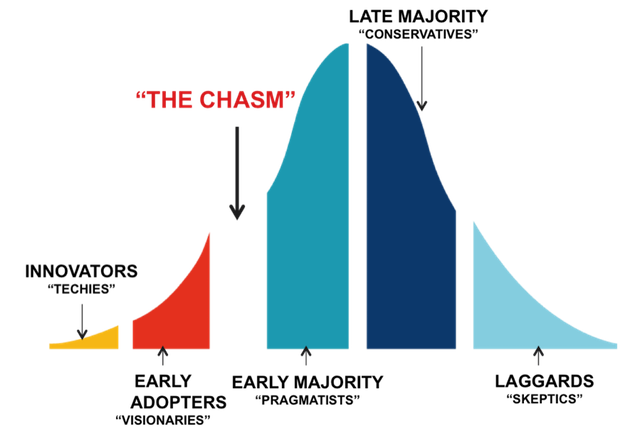
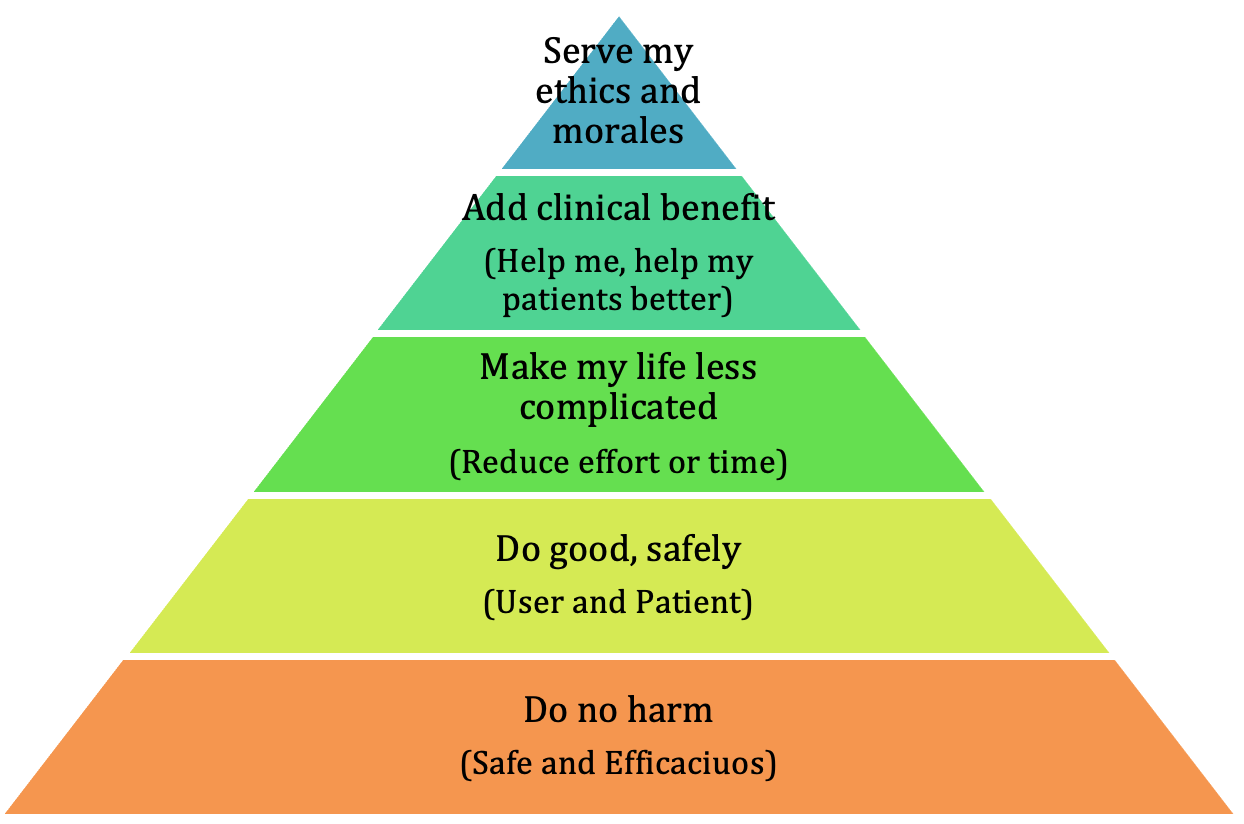
Persona shifts – When the majority of your audience has shifted right along the adoption curve the persona of the target audience will shift towards a more conservative one. Language and strength of clinical evidence, colors, and media channel will need to be tweaked to optimize the impact you will have on the new audience.
- Innovators / Pioneers
- Early adopters
- Early majority
- Late majority
- Laggards
Clinical evidence shifts – the focus, driven by good, or, no so good, results toward a different clinical target, or a new class of patient. Perhaps you discover that your economic story is stronger than you had originally thought.
- KOL editorials /Testimonials
- Posters
- Single-center case series
- Controlled clinical trials
- Multiple controlled trials
- Meta-analysis
- Economic data
Launch phases – depending on what phase in the initial launch you are in your goals, focus, targets, and media channel may well need to be optimized to a new type of customer.
- Clinical trial recruiting of PIs
- User trials
- Controlled launch
- Limited launch
- General launch – primary geography
- Repeat for each region of the World into which you are launching.
Other aspects of the product life cycle that can trigger the need for a new campaign are:
- Competitive response
- Pricing shifts
- Contracts being secured
- Reimbursement changes
- Device enhancements
- Complaints
- FDA actions
- Achieving a number one share position
- Therapeutic/clinical practice changes
- Major environmental changes
- Regulatory policy or guideline shifts
A cautionary note
As mentioned in several earlier posts “message congruency” is critical.
You can’t isolate one group of customers from another completely. Your message optimization should not cause dramatic changes and  the core value position must remain in tack. So be careful. The one time you can be a bit bolder in your changes is where you can control access to an “event”.
the core value position must remain in tack. So be careful. The one time you can be a bit bolder in your changes is where you can control access to an “event”.
As a rule of thumb, I try to maintain a campaign for 18 months. With digital marketing it is so much easier to change the message you will be tempted to jerk the message around too often. Don’t. Remember the fundamentals you need to validate every messaging change you make before it is released to the target audience.
The only thing worse than not occasionally optimizing the messaging, is changing it so often or so dramatically the device loses its core identity, and you begin to confuse the clinician.
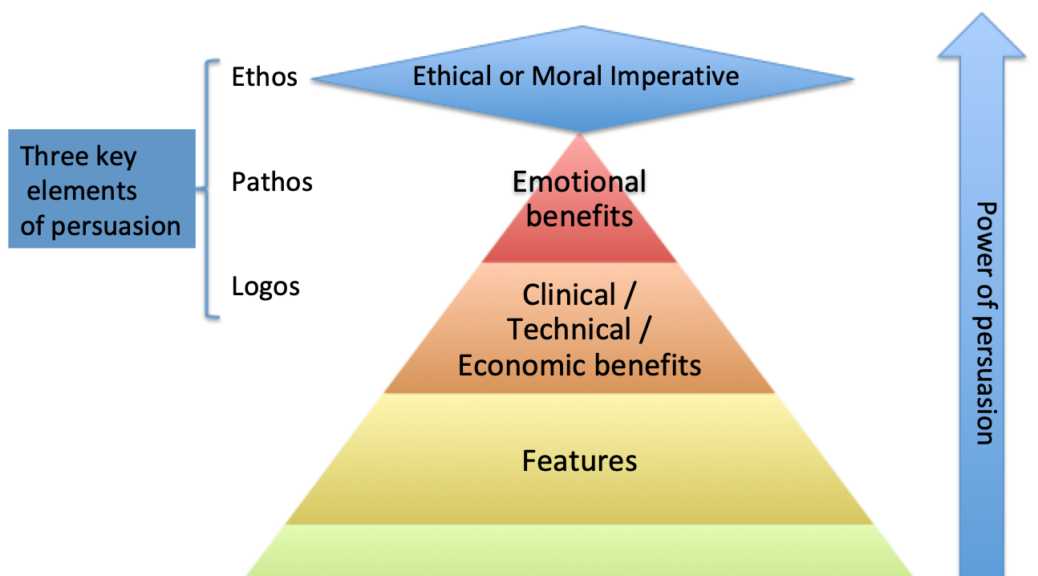
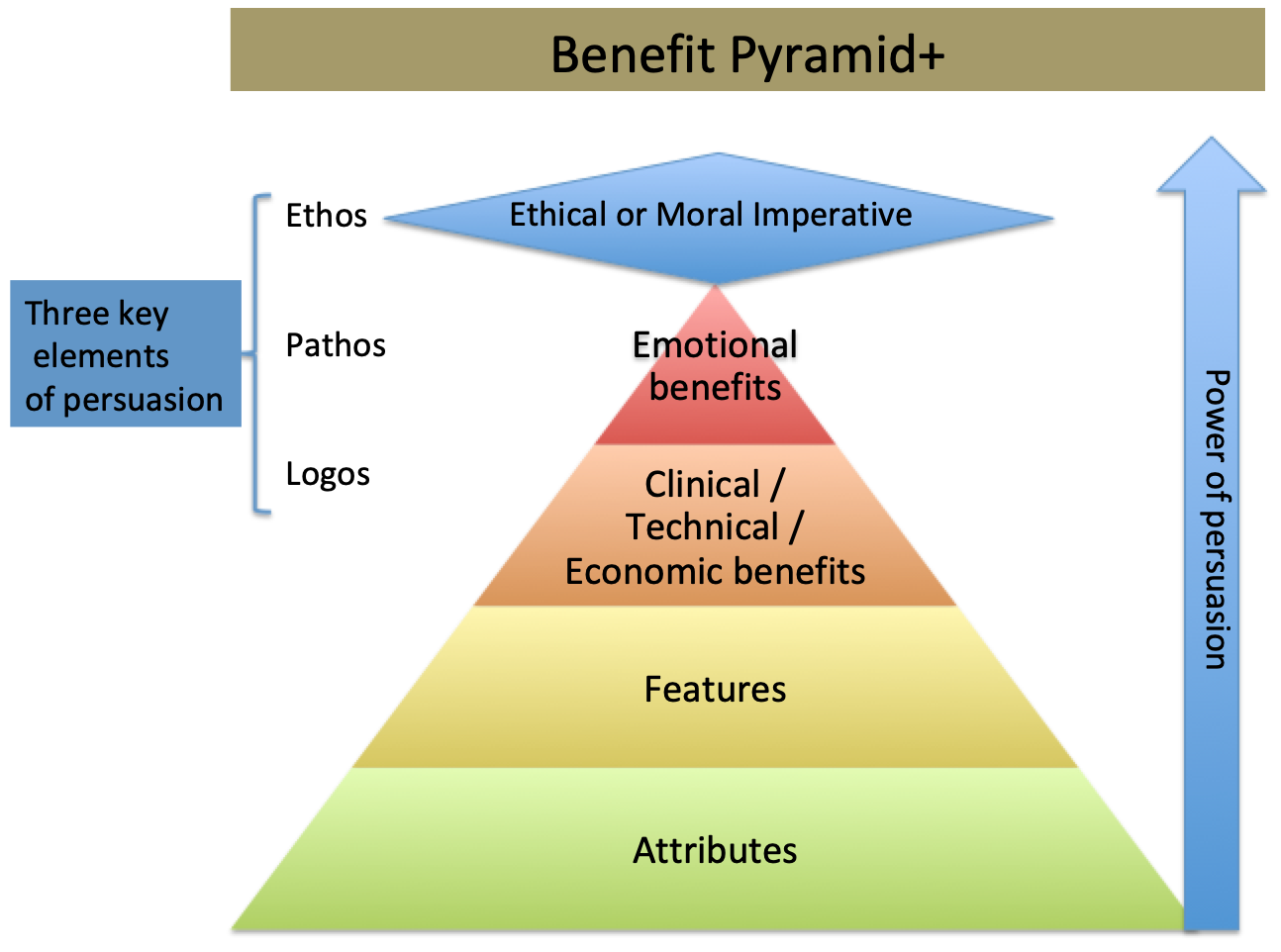
Examples of optimization that might focus a campaign –
- First – to – Best
- Best – to – Gold standard
- Innovative – to – Proven
- Premium – to – The highest value
Metaphor –
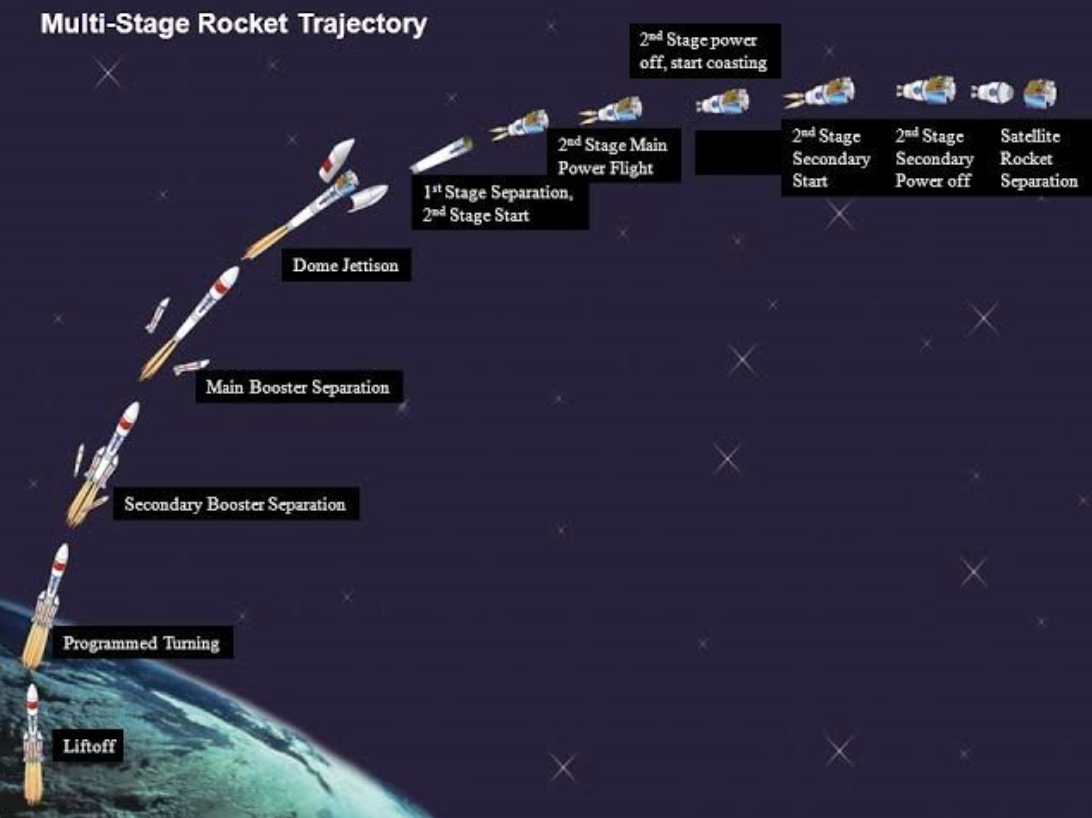
We have often thought of launching a rocket as a metaphor for launching a new product. I have only written about lift-off. To reach outer space, often booster stages are required, second and third stages might be needed to break free of the earth’s gravity. Depending on the size of the payload, more stages or booster engines may be required. The same goes for new products.
Metrics –
Another lesson from NASA for us is that if we don’t have a course to follow, we can’t know if we are on, or off, trajectory. Set your course pro-actively, check it periodically, and then correct by firing your navigational boosters, gently.
Challenge –
Here is a challenge for you, if you strive to become an even better product manager or up-stream marketer.
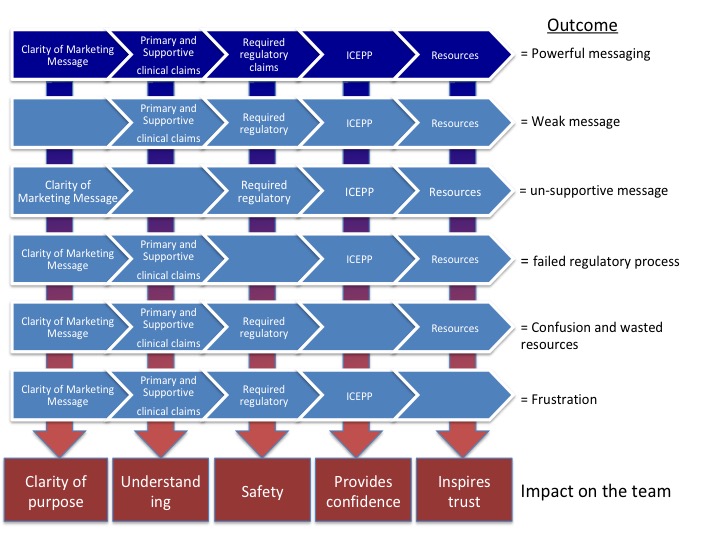
The next time you are planning a launch, create a campaign progression map. Imagine covering the entirety of the product’s life cycle.
Lessons:
- Uncertainty is not to be feared, be bold and think the whole product life cycle through from the very beginning to the end.
- Targeted customer personas shift with time and distance along the adoption curve.
- Having effective metrics is critical.
“Experience is what you get, right after you need it most.”
Make it a great day!
Tim Walker
Tim Walker is the Principal Consultant for The Experia® Group, a consulting firm specializing in providing experience and expertise during critical device commercialization phases to increase the probability of success. One-on-One, or, team coaching is available.
www.theexperiagroup.com.
Contact, The Experia® Group for a free 30-minute consultation to determine if 30+ years of experience can contribute to your success. [email protected]
© 2021, The Experia® Group, LLC
[1] Available on Amazon