From time to time, great events shape, speed, or cause change to our World. You know the list as well as anyone, war, famine, pandemics, climate shifts, geological shifting of the tectonic plates each of these significant upheavals has set in motion many dimensions of change. Biology, technology, and society evolve in an adjustment to the new parameters of the new age that results from the energies released during these global events.
Whether COVID-19 is one of these upheavals or not is yet to be  seen. But reflecting on how it might impact the world of medical device marketing, it begs the question, are we ready?
seen. But reflecting on how it might impact the world of medical device marketing, it begs the question, are we ready?
The energy that will most likely drive this resulting change to our time will be fear. While fear is a primal motivator for action and is key to survival, it often lends itself to noise and overreaction. It creates the opportunity for those less honorable to take advantage of those not prepared.
Let’s all keep our priorities straight, facts matter, safety first, regulatory processes can be useful, oversight is proper, in a pure capitalist system the safety of each member of society is equal.
Tactics will change, strategies not so much
The strategies won’t change, the tactics will shift away from 80% face-to-face selling toward the digital, or virtual worlds.
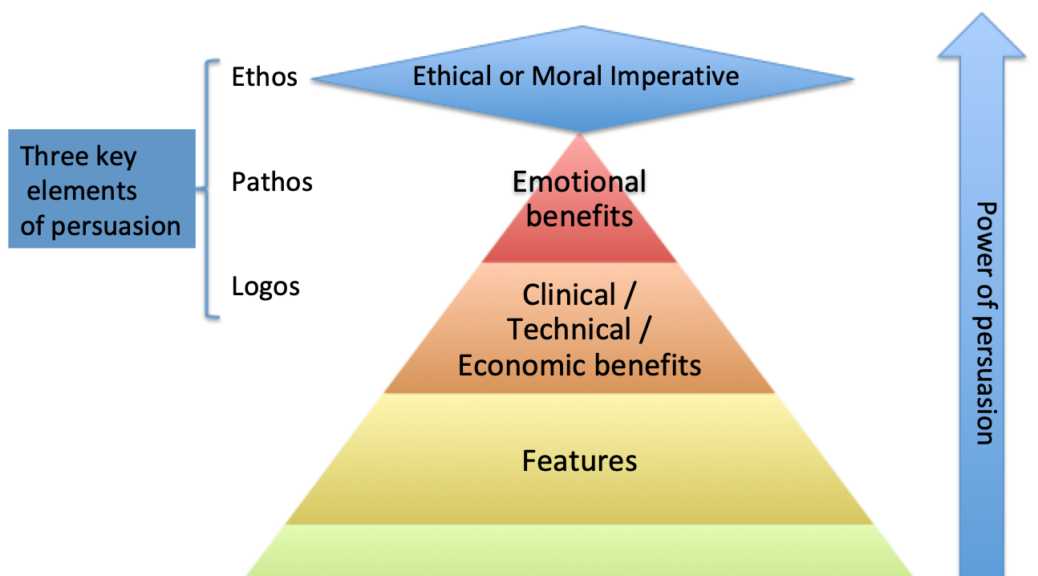
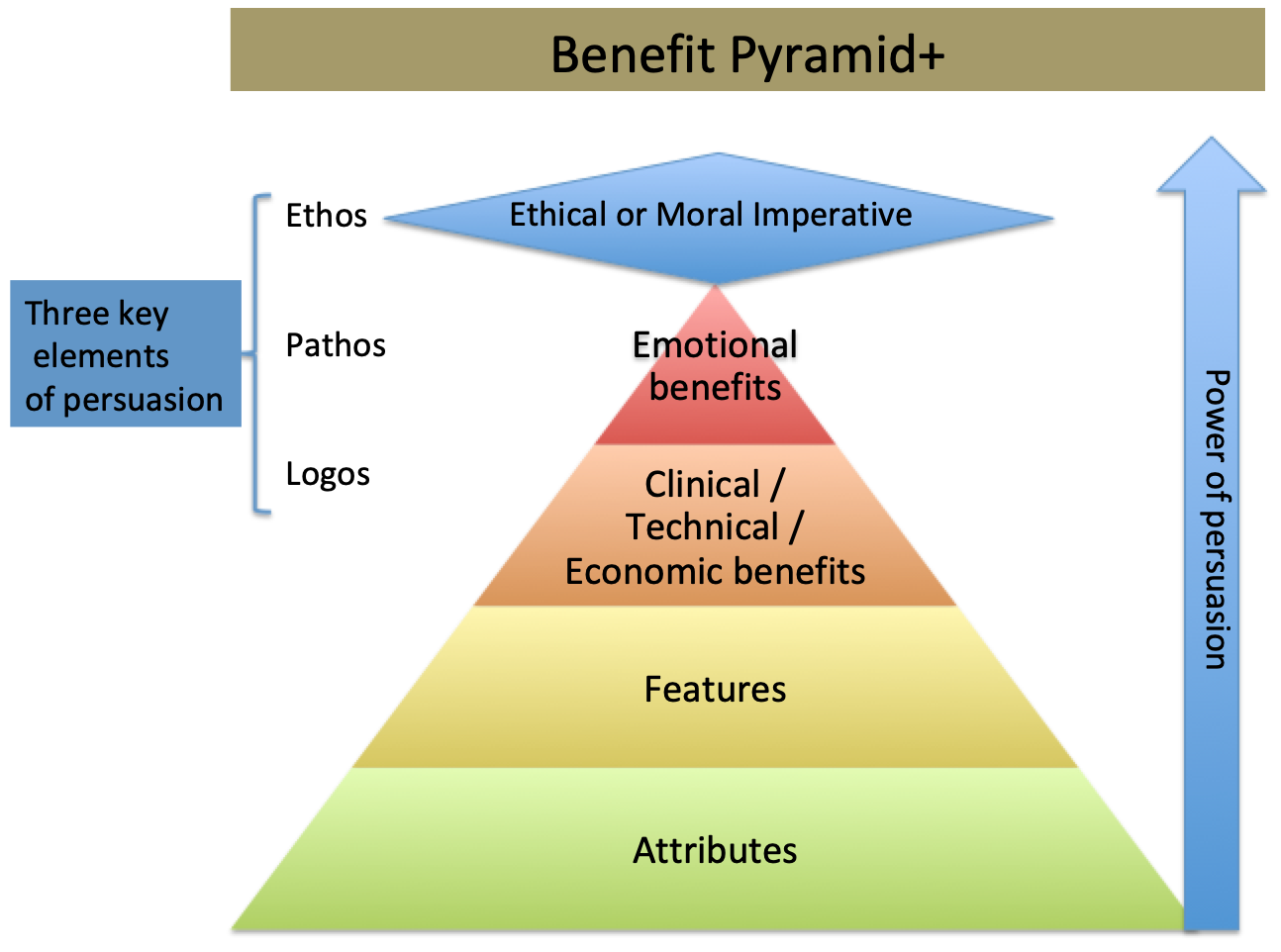
One Potential Impact
For a decade, the desire to use the Internet more as a means of influencing behaviors of clinician purchasing, or product selection, has been stunted by the overwhelming majority of clinicians who are resistant to change. Their resistance to change is not only relating to the adoption of new best practice therapy but extends to the way they get information about new products.
Based on the last survey The Experia Group conducted, clinicians identified the top three ways of receiving new product information as 1) colleague recommendations, 2) congress trade show symposium and booths, and 3) from trusted manufacture representatives.
Additionally, the way clinicians prefer to evaluate new products is by having a trained clinically oriented representative of the company present in the hospital or procedure room so that they are present to answer any questions that might come up.
There have been several environmental pressures to move toward digital or remote training on new products. Among these pressures are The Sunshine Act, HIPPA, the restrictions on access to hospitals, the number of administrative processes that are now required to get approval to trial new products, including the expanded use of IRB’s, to name just a few.
Now that face-to-face contact between industry and clinicians has been reduced dramatically, and rightfully so, the COVID-19 pandemic may be the last push needed to total re-orient the selling, evaluation, and training processes for new product awareness and adoption of medical technology.
Are you ready?
What new skills will we all need to develop if this new World comes into existence?
Just off the top of my head:
-
-
- Community building
- Hosting webinars
- Digital copywriting
- Educational content creation
- Remote customer engagement and interaction
- Video content editing
- Technical skills in managing and utilizing apps and facilitate communication
- Empowering your field organization to use the new skills
- Label copy review processes may need to modified for speed w/o giving up the proper level of controls.
-
One challenge that I don’t see an emerging answer for is, how to teach clinicians to optimize their ability to learn remotely.
Perhaps the more significant challenge is how to teach, feel, and touch that so many products require, remotely.
For me, the above list of skills to learn are all tactics and abilities. The strategies for success don’t change. The choice of tactics may be more digitally biased.
Breathe
Not all of the historical sales, education, training, and support techniques will go away. New hybrid approaches will evolve. Regardless of your current skill level, you need to develop a higher level of awareness and execution capability as a person and as an organization. Even if everything settles down after the pandemic passes, what have you lost by learning a new skill set?
Typically, I would offer a solution to the issue. Sorry, but I don’t have one just yet. For me, I have some learning to do.
Go to www.theexperiagroup.com, where I will be building a resource list (over time) as I develop or refine new skills.
Lessons:
1) Never stop learning
2) Always keep your environmental scans up and running
3) It is ok to go slow, to go fast, but you need to start
“Experience is what you get, right after you needed it most.”
Make it a great day!
Tim Walker, MBA
Tim Walker is the Principal Consultant for The Experia Group. A consulting firm that specializes in providing expertise and experience during critical device commercialization phases to increase the probability of your success.
One-on-one and team coaching are available.
www.theexperiagroup.com. Contact The Experia® Group for a free 30-minute consultation to determine if 30-years of experience can contribute to your success. [email protected].
© 2020, The Experia® Group, LLC
Available from Amazon.