The Set Up
I have found that there is often a disconnect between the desired Marketing Messaging and the Clinical Evidence proof that has been created in support of the product. Many product managers are caught between what they want to say vs. what they are allowed to say. This sets up a potential conflict that could lead to “off-label” claims.
The Problem
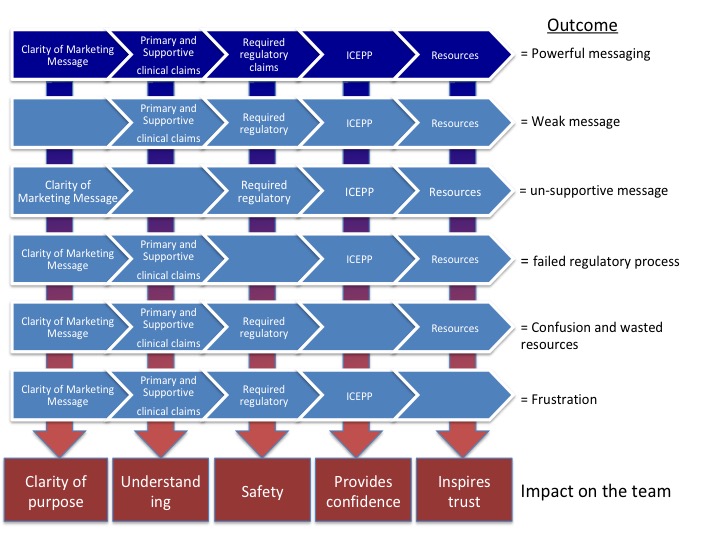
When there is a disconnect between messaging and proof points a number of outcomes may or will occur:
- The message is weak
- Un-supported claims – misleading statements
- Failed regulatory process
- Confusion and wasted resources
- Frustration
What causes the disconnect?
There are a number of reasons this disconnect might occur:
- Lack of an integrated Commercialization strategy
- The Clinical Affairs group acting to serve the minimal required Regualtory Strategy
- Marketing not providing input to Clinical Affairs before the evidence collection begins
- Marketing not knowing what they want to say about the product
- A desire to minimize cost
None of the above reasons are good ones. We have talk about the product managers role as being the keeper and communicator of the vision for the new product launch. This has to include the development of a clinical evidence plan.
What to do to prevent the disconnect?
Create a Clinical Evidence Plan – a clinical evidence plan is the thought process and document that connects your marketing message to the clinical trial/study development goals with the regulatory strategy. As with all commercialization processes there are natural tensions between the four roles that surround clinical evidence development. Getting to a consensus won’t be easy. The gains are worth the effort.
How do I create a clinical evidence plan?
There are thirteen steps to creating the plan. I’ll list them here for you, but it may not be clear to you how to actual complete each step. Steps 5-12 are particularly difficult and require some experience to do them well.
Plan creation steps (assume that you will need a working group of marketers, clinical affairs specialists, medical affairs specialists, and regualtory affairs specialists)
| Step | Description | Type of step |
| 1 | Strategic marketing message (clarity is required on primary claims) | Input |
| 2 | Proof points from messaging | Extraction |
| 3 | Brain storm support claims | New input |
| 4 | Brain storm desired future claims (outside current messaging) | New input |
| 5 | Load all desired claims into a spreadsheet (Steps 2,3,4)(leave a comment to this post with your e-mail address for a free spreadsheet template) | Team leader action |
| 6 | Populate the spreadsheet with the claims and all details
Nature of the claim, clinical end point, evidence level required, study type, study description, regulatory requirement, sample size, number of investigators needed, etc. |
Team event(s) |
| 7 | Define scoring scales 5-1 for each impact variable
· Duration of study score · Total cost score · Magnitude of market impact score · Likelihood of success score Selecting weighting for each variable |
Team event |
| 8 | Have an expanded group score each variable, independantly | Individual input |
| 9 | Collate and compile the results from all receiptient (best to do frequency plots and averages for all variables) | Team leader action |
| 10 | Review scoring and reach concensus on each score, don’t just use the average score. | Team event |
| 11 | Calculate the impact scores (Duration x weight) x (Total cost x weight) x (Magnitutude market impact x weight) x (Likelihood of success x weight) = Combined impact score | Team leader action |
| 12 | Apply team judgement to the scores and prioritize | Team event(s) |
| 13 | Publish results | Team lead and team |
Cautions: Scales and weights will drive the prioritization; spend enough time on these two sets of decisions.
The Benefits of an Integrated Clinical Evidence plan are:
- Powerful messaging
- Team alignment
- Effective use of resources
Lessons:
- Be proactive and lead
- Know what you want to say about the product before you start the clinical evidence collection process
- Build consensus on the best approach to develop clinical evidence
“Experience is what you get, right after you need it most.”
Make it a great day,
Tim Walker
Tim Walker is the Principal consultant for The Experia Group. A small consulting firm that specializes in providing experience and expertise during critical device commercialization phases to increase the probability of success. www.theexperiagroup.com. Contact The Experia Group for a free 30-minute consultation to determine if 30-years of experience can contribute to your success.
Copyright 2018, The Experia Group®, LLC