Prelude
With the creation of the STeP process the FDA finally, after 45-years, signals that they are proactively trying to fill the implied  mandate from Congress to protect the US population by ensuring that safety is proactively promoted in the medical device space.
mandate from Congress to protect the US population by ensuring that safety is proactively promoted in the medical device space.
Set-up
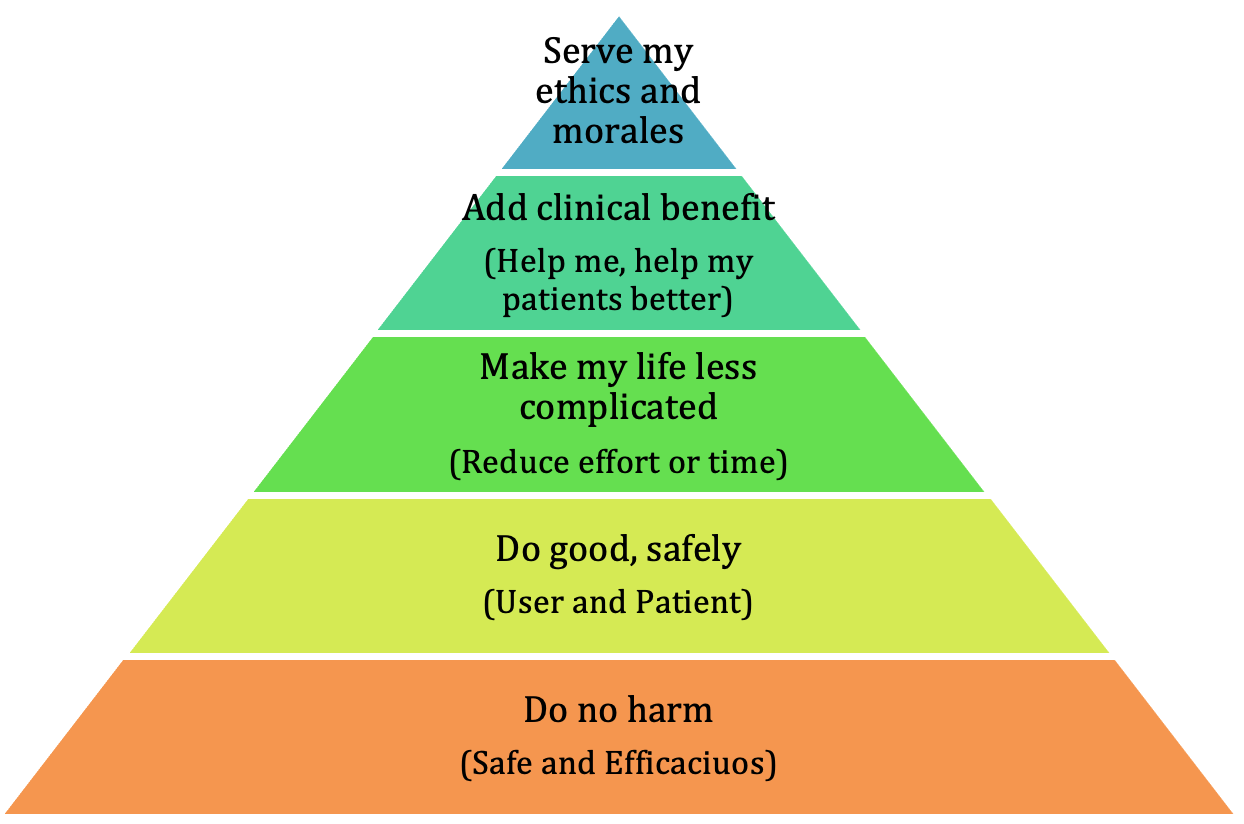
Safety has always been a prime motivator for change within the human population. When I think about the motivators for physicians to change their purchasing (use) behaviors I reflect on a model that we have all seen before, Maslow’s hierarchy of needs. Modifying Maslow’s labels for the five levels to those of a physician’s we might see something like this. Of course, there are more and different ways of saying them, but essentially this is what motivates physicians to buy or try new technology.
The STeP Process
The STeP process allows the FDA to expedite the review of devices that have the potential to significantly improve safety. If you are defining a device that will:
- Reduce the occurrence of a known serious adverse event
- Reduce the incidence of a known failure mode
- Reduce a known use-related hazard or use error
- Improve the safety of another device or intervention
You should factor in the STeP time and cost savings into the justification for proceeding with the R&D project. Having your product designated as a STeP device has real messaging and PR potential. More importantly, as you consider how to prioritize competing products for your new product road map, the STeP designation should provide additional consideration.
Note: The FDA has prioritized Breakthrough Technology above the STeP program for resource allocation. Breakthrough Technology program is mandated by Congress.
Conclusion
Yes, the STeP program is significant to medical device marketers. To learn more visit [email protected]
Lessons:
- Know the regulations so you can get the most out of your partnership with the FDA
- Patient safety is not assumed, it must be advocated for by every department and person, the FDA just made that advocacy easier for you.
- Scan the grand environment constantly for changes that can help and sometimes detract from your product line success.
“Experience is what you get, right after you need it most.”
Make it a great day!
Tim Walker
Tim Walker is the Principal Consultant for The Experia® Group, a consulting firm specializing in providing experience and expertise during critical device commercialization phases to increase the probability of success. One-on-One, or, team coaching is available.
www.theexperiagroup.com.
Contact, The Experia® Group for a free 30-minute consultation to determine if 30+ years of experience can contribute to your success. [email protected]
© 2021, The Experia® Group, LLC
[1] MED DEVICE ONLINE, Mark Durivage, January 25, 2021