The Set Up
Over the past two-years I have had the good fortune to work directly with five medical device start-ups. Through my mentoring volunteer work I have also spent time with seven other start-ups, mostly medical devices.
A key observation for me is that being an entrepreneur is a spiritual journey. It is a calling that you can’t hide from. It is also a journey that you can’t take alone. No matter how broad your background is in all the disciplines of commercializing medical devices you still can’t know it all.
Why? Because time marches on! The experience you may have gain in sales or R&D or mfg. will grow stale with time. The advice you may be getting could be dated. I want you to succeed, so here is what I think you need to realize.
What Has Changed?
From a start-ups perspective, in most cases, the days of bootstrapping a finished product are gone. The need for high energy, high impact launches, and the  capital to execute them, all but exclude slow rolling the post-launch of a new product. Why high-energy, high-impact launches? It takes tremendous energy to break through the noise of the big players. Similar to the launch of a space rocket, it takes tremendous energy to break free of Earths gravity.
capital to execute them, all but exclude slow rolling the post-launch of a new product. Why high-energy, high-impact launches? It takes tremendous energy to break through the noise of the big players. Similar to the launch of a space rocket, it takes tremendous energy to break free of Earths gravity.
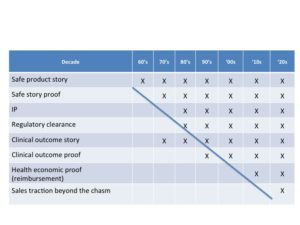
Take a look at the following chart to see how acquirers have changed their expectations of potential device acquisitions over the last six decades.
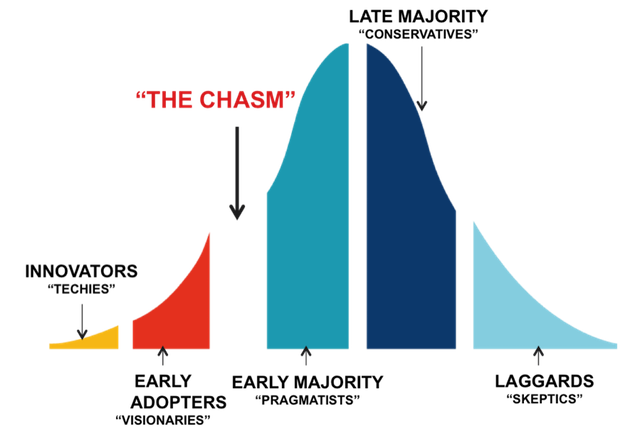
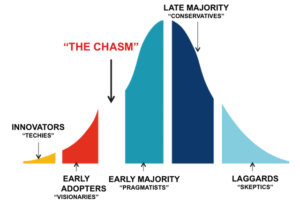
A simple story is not enough, a product is not enough. In the decade of the 2000’s potential acquirers want all the risk removed. They only want to have to apply money and their high-powered sales channel to scale success. This means that you have to be prepared to take the device all they way through to commercial success. Commercial success does not mean that a few pioneering physicians have made it work. It doesn’t mean that you completed your clinical trial. It simply means that you have a validated “equation” for successfully selling into the early majority, proof that you have leapt the chasm.
What are the components of the “equation”?
- Strong messaging (connects on an emotional level)
- Sales process that works (80% of the time)
- Believable value proposition
- Routine clinical success (utility is delivered w/o a lot of hoops or work-a-rounds)
- Clinical and economic evidence packaged professionally
- Satisfied customers who are re-ordering
What Hasn’t Changed?
Over the past six (6) decades almost every aspect of commercializing medical devices has changed. The aspects that have not changed are the core principals that I have referred to before.
- Engage with you customer

- Safety, safety, safety (no short-cuts)
- Quality, quality, quality (no short-cuts)
- Deliver complete utility with hard value
- Make the regulatory bodies your friend
- There is no single formulae for success, only guidelines
Take a-way
In today’s world of medical devices, entrepreneurs and inventors need to have a longer-term vision for their companies. The days where a patent, or regulatory clearance will trigger an exit are all but gone. Too often the big players have not realized their hopes for gains from an acquisition. Often because the “equation” wasn’t thoroughly validated in the real market.
The deeper you go into the commercialization process the more help you are going to need. You can’t do it alone. You need a team with skills that are current and relevant.
“Experience is what you get, right after you need it most.”
Make it a great day,
Tim Walker
Tim Walker is the Principal consultant for The Experia Group. A small consulting firm that specializes in providing experience and expertise during critical device commercialization phases to increase the probability of success. www.theexperiagroup.com. Contact The Experia Group for a free 30-minute consultation to determine if 30-years of experience can contribute to your success.
© 2016, The Experia Group, LLC