The Set Up
I recently attend the VIVA2016 conference held in Las Vegas. I ran into a number of marketing wizard there. Several of them challenged my opposition to using the MVP product strategy for medical devices.
So I will be breaking into the chain of Portfolio development topics and take this post to re-frame some of the points that I made regarding MVP.
Clarification
Let me be clear, I am not opposed to the use of the MVP concept. It can be very valuable. My concern is in how it is used by those who are not well schooled in the device word. I have found with many inventor / entrepreneurs (brilliant people all of them) that they latch on to these concepts at a superficial level and could be setting themselves up for failure or worst yet, hurting people.
What is Meant by MVP?
When Eric Ries used the term for the first time he defined it this way: “A Minimum Viable Product is that version of a new product which allows a team to collect the maximum amount of validated learning about customers with the least effort.” Nowhere in this definition does he mention commercial viability. For the device world this may well be represented by a clinical trial.
The Caution?
We have to be careful when extending management (marketing) concepts across markets and industries. While there is a huge opportunity to learn from concepts formulated in other markets, sectors, industries and disciplines we can’t assume that they can be directly applied. We have to be good Marketers and review all the assumptions and environmental consideration and then apply them in our context.
We have seen where the application of Software or App development thinking has failed in the medical device space. How one manages the risk of failure is very different when the result is death or serious injury vs. being disappointed. MineCraft, the now very popular video game was launched commercially after only 6 weeks of development and was barely functional. It was however a great application of MVP. It served as a proof of concept. It offered a complete game, just not a very finished one. The risk of failure in MineCraft did not include death or serious injury to a real human.
One example (not a perfect one) of this is Theranos. They produced an MVP that failed to offer complete clinical utility (clearly an over simplification, but illustrative none-the-less). We will leave the why to others. Great concept, great story, great funding but the product just didn’t offer a complete clinical solution to the problem.
But a great quote from the reporter that broke the story that appeared in Vanity Fair’s article on Theranos, …Carreyrou was simply emboldened. “It’s O.K. if you’ve got a smartphone app or a social network, and you go live with it before it’s ready; people aren’t going to die,” … “But with medicine, it’s different.”
The MVP Concept, modified for devices
In Medical Device Development (MDD), the V in MVP is huge and unyielding. In today’s world the term Viable is getting bigger. It could be expressed as:
V = ƒ(V,S,R,CCU,C,$)
V = viable
S = safe
R = regualtory cleared or approved
CCU = offers a complete clinical solution
C = the promise of Cost neutrality
$ = a pathway to reimbursement
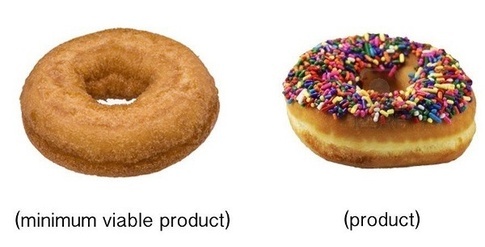
What do I mean when I say “complete clinical utility (CCU).” Once you get to the clinic you need to offer “complete clinical utility”. You must completely (minimally) solve the clinical problem that you were attacking. If you don’t you have delivered no real value to the clinician. By all means leave the sprinkles and glaze off, but give them a whole doughnut.
(minimally) solve the clinical problem that you were attacking. If you don’t you have delivered no real value to the clinician. By all means leave the sprinkles and glaze off, but give them a whole doughnut.
What I do find helpful is the idea of “fast to failure” referring to prototyping, at all three levels: concept, feasibility, and design. These prototypes can be partial, abstract, bread boards through Verification testing samples, but they don’t get near a patient.
A Different Way to Think about MVP
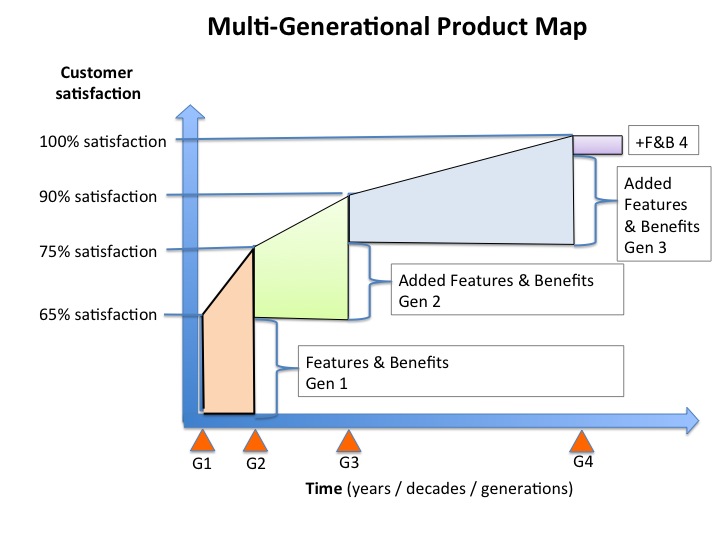
Fully integrated multi-generational product planning. A documented, data driven, customer focused pathway to delivering 100% customer satisfaction in steps. This type of plan can span decades as with the Left Ventricular Assist Devices (LVAD). Or, it can take just a few years as with the SMART Stent.
Call it what you will, MVP, CCU, multi-generational planning, doesn’t matter. Whatever you do, make it safe.
“Experience is what you get, right after you need it most.”
Make it a great day,
Tim Walker
Tim Walker is the Principal consultant for The Experia Group. A small consulting firm that specializes in providing experience and expertise during critical device commercialization phases to increase the probability of success. www.theexperiagroup.com. Contact The Experia Group for a free 30-minute consultation to determine if 30-years of experience can contribute to your success.
© 2016, The Experia Group, LLC